Biostatistics and Clinical Epidemiology
About the node
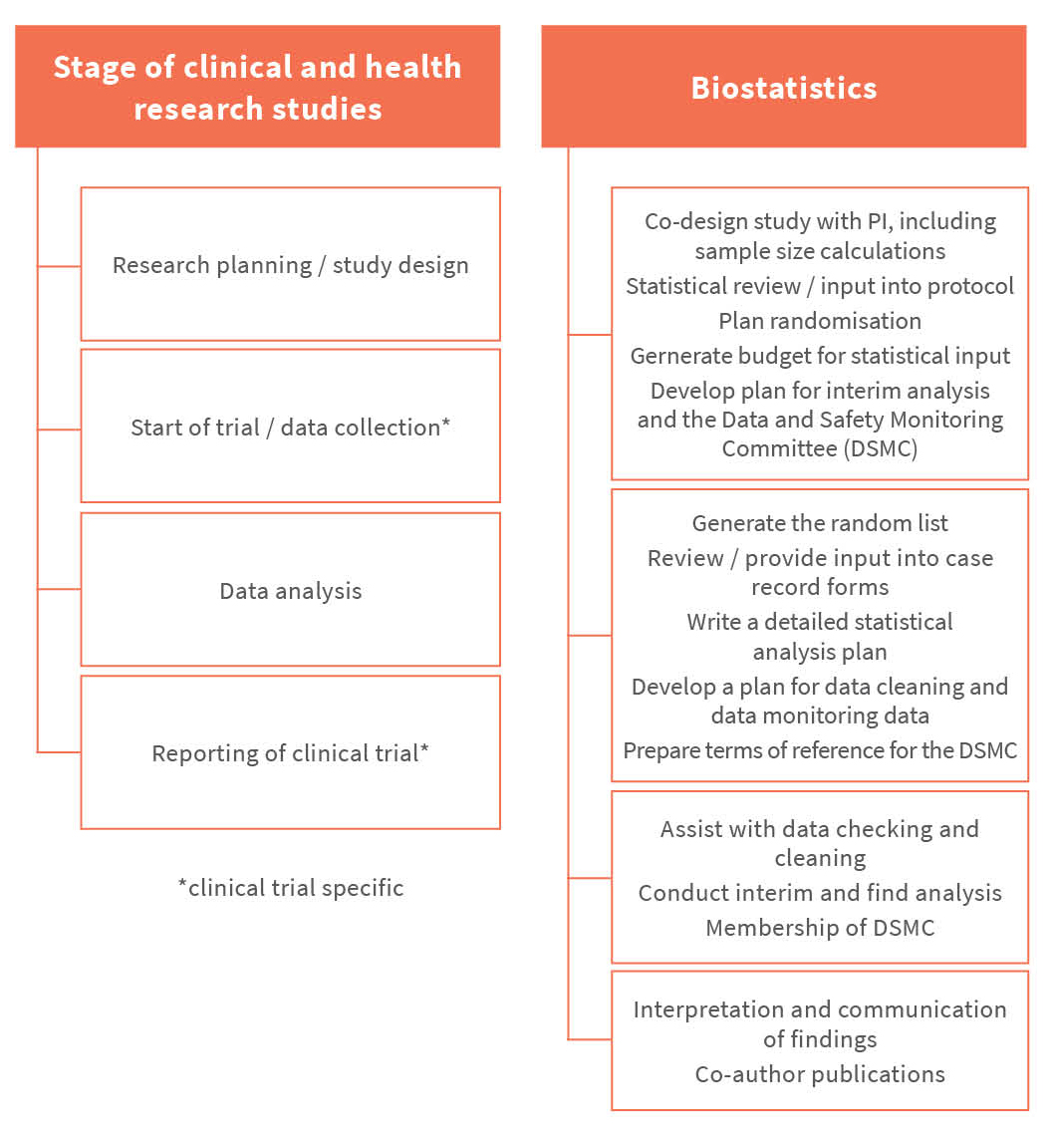
Biostatistics and clinical epidemiology are critical to ensure the success of clinical and health research studies. Biostatistical as well as epidemiological skills are required throughout all stages of the design, conduct and analysis of a research study. An essential component of Biostatistics is the sound application of appropriate statistical methods. This is complemented by knowledge and skills in the design of both clinical trials (see figure below) and observational research studies (see figure below), as well as an ability to appropriately report and interpret data from clinical and health research studies. The Biostatistics and Clinical Epidemiology Node offers this expertise to health researchers affiliated with the University of Melbourne.
Our MISCH biostatisticians/epidemiologists provide expertise in the following:
Design stage - Development of the research question(s), selection of study design, sample size calculation, writing of grant applications, study protocol development, ethics applications, statistical analysis plan creation.
Execution stage - Review data collection forms and database design, generate randomisation lists, quality control of data collection procedures (including ethical handling of data), contribution to data safety monitoring committee reports.
Completion stage - Support data cleaning and reproducible data processing, conduct statistical analysis (especially where requiring skills to perform complex analyses), preparation of written summaries (including graphical and tabular displays) of statistical analyses for publication in health-related
journals or professional reports, including sound interpretation of statistical findings.
Translational stage - Interpretation and effective communication of research findings, leading to the development of policies and guidelines.
A FAQ brochure can be downloaded HERE